Reducing the Global Impact of Diabetes
Diamune’s cutting-edge and proprietary Fc-fusion protein technology has allowed the development of products that are changing the way diabetes is treated and prevented.
AKS-107 offers a prevention-first approach to type 1 diabetes, intervening at the earliest stage (Stage 1) before the disease commences. AKS-440, a once-weekly basal insulin therapy, transforms type 2 diabetes management by improving convenience and control with fewer injections.
Diamune Pipeline
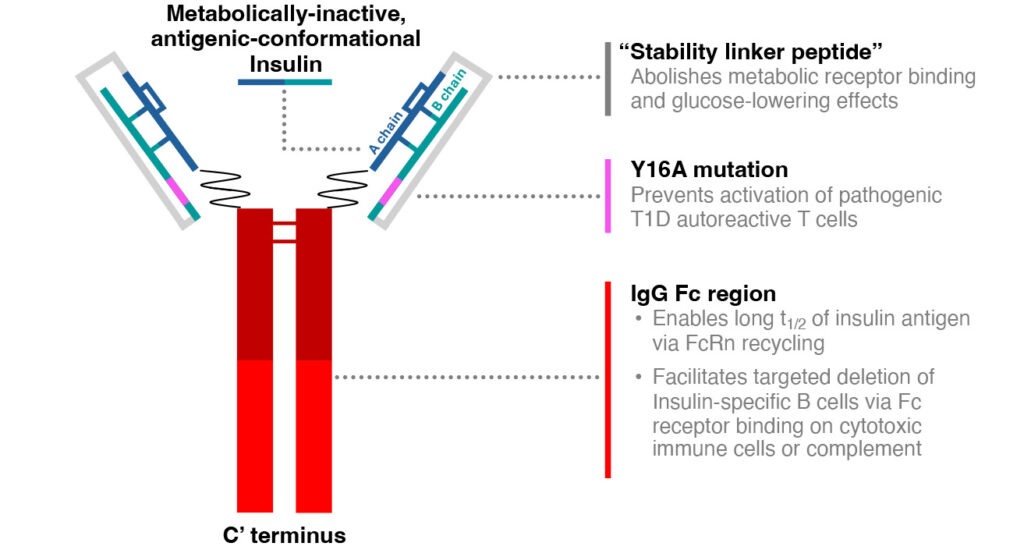
AKS-107 is an Fc-insulin fusion protein designed to interrupt the process leading to insulin autoimmunity. Diamune Therapeutics, Inc. has licensed this patent from Akston Biosciences Corporation to continue the development that was begun with a partnership with the Helmsley Charitable Trust and the National Institutes of Health (NIH) in the early development stages.
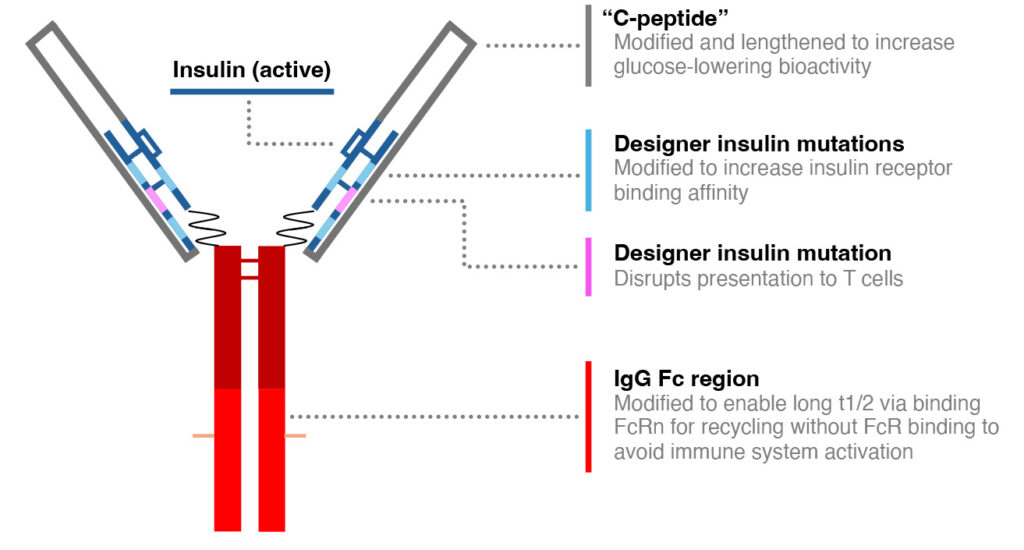
AKS-440 is a novel human insulin candidate based on an Fc-fusion protein platform, intended to be a once-a-week injectable diabetes therapy. Diamune Therapeutics, Inc. has licensed this patent from Akston Biosciences Corporation to continue the development of this therapy. It is currently in pre-clinical development.